Mainly antiparallel beta sheets (segregated alpha and beta regions)
consists of one alpha-helix and 4 strands of antiparallel beta-sheet and contains the catalytic triad Cys-His-Asn
the constitute families differ by insertion into and circular permutation of the common catalytic core made of one alpha-helix and 3-strands of beta-sheet
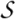 uperfamily
uperfamily
displays a minimal thiol-protease fold and its catalytic triad; potential redox regulation: the catalytic cysteine can form a disulfide bond with an N-terminal cysteine